[ad_1]
Bluebird Bio confirms FDA approval of Zynteglo
bluebird bio (BLUE) announced the U.S. Food and Drug Administration has approved Zynteglo, also known as beti-cel, a one-time gene therapy custom-designed to treat the underlying genetic cause of beta-thalassemia in adult and pediatric patients who require regular red blood cell transfusions.
“Due to the complex nature of gene therapy, Zynteglo will be available exclusively at Qualified Treatment Centers, which are carefully selected based on their expertise in relevant areas such as stem cell transplantation, cell and gene therapy, and beta-thalassemia; and receive specialized training to administer Zynteglo.
Information on bluebird’s QTC network, as well as personalized support focused on the needs of each patient throughout their treatment journey and information on insurance coverage and access will be available through bluebird’s patient support program,” the company stated.
“The FDA approval of Zynteglo offers people with beta-thalassemia the possibility of freedom from burdensome regular red blood cell transfusions and iron chelation, and unlocks new possibilities in their daily lives.
After more than a decade of research and clinical development, and through the perseverance of clinicians, patients, and their families, the approval of Zynteglo marks a watershed moment for the field of gene therapy.
As the first ex-vivo lentiviral vector gene therapy approved in the U.S. for the treatment of people with beta-thalassemia, we are ushering in a new era in which gene therapy has the potential to transform existing treatment paradigms for diseases that currently carry a lifelong burden of care,” said Andrew Obenshain, CEO of bluebird bio.
Expensive Treatment
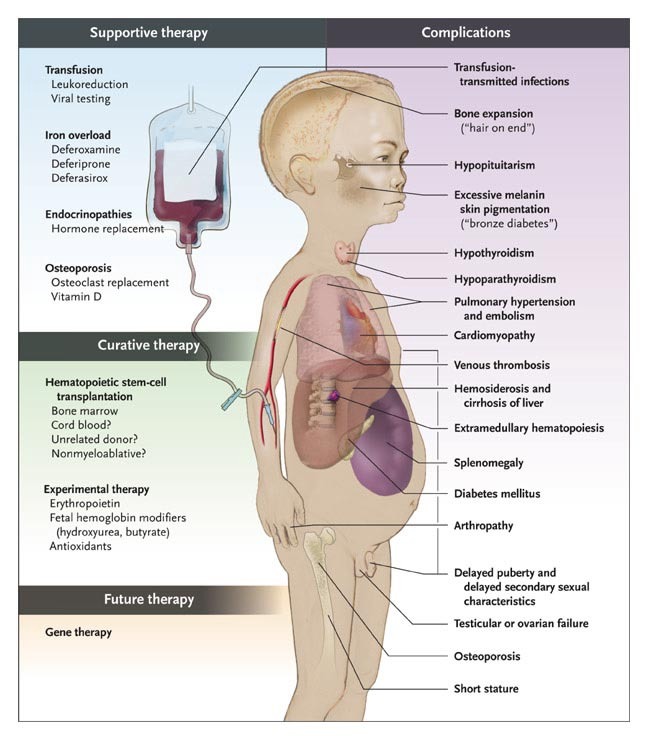
The lifetime cost of medical care for a patient with transfusion-dependent beta-thalassemia can reach up to $6.4M in the U.S. and the average total health care cost per patient per year is 23 times higher than the general population. bluebird estimates that there are approximately 1,300-1,500 individuals with transfusion-dependent beta-thalassemia in the U.S.
” bluebird has set the wholesale acquisition cost of Zynteglo in the U.S. at $2.8M, in “recognition of its robust and sustained clinical benefit demonstrated in clinical studies and its potential to alleviate a lifetime of health care costs associated with regular RBC transfusions and iron management.” Tom Klima, chief commercial and operating officer of bluebird bio, said.
STOCKWINNERS
To read timely stories similar to this, along with money making trade ideas, sign up for a membership to Stockwinners.
This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility.
[ad_2]
Image and article originally from www.stockwinners.com. Read the original article here.